Heat energy is released by the substance. Releases latent heat With each phase change a specific amount of latent heat is released or absorbed.
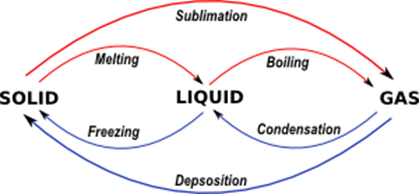
Change Of State Of A Matter Melting Fusing Evaporation Condensation
9 Latent Heat Examples In Daily Life Studiousguy
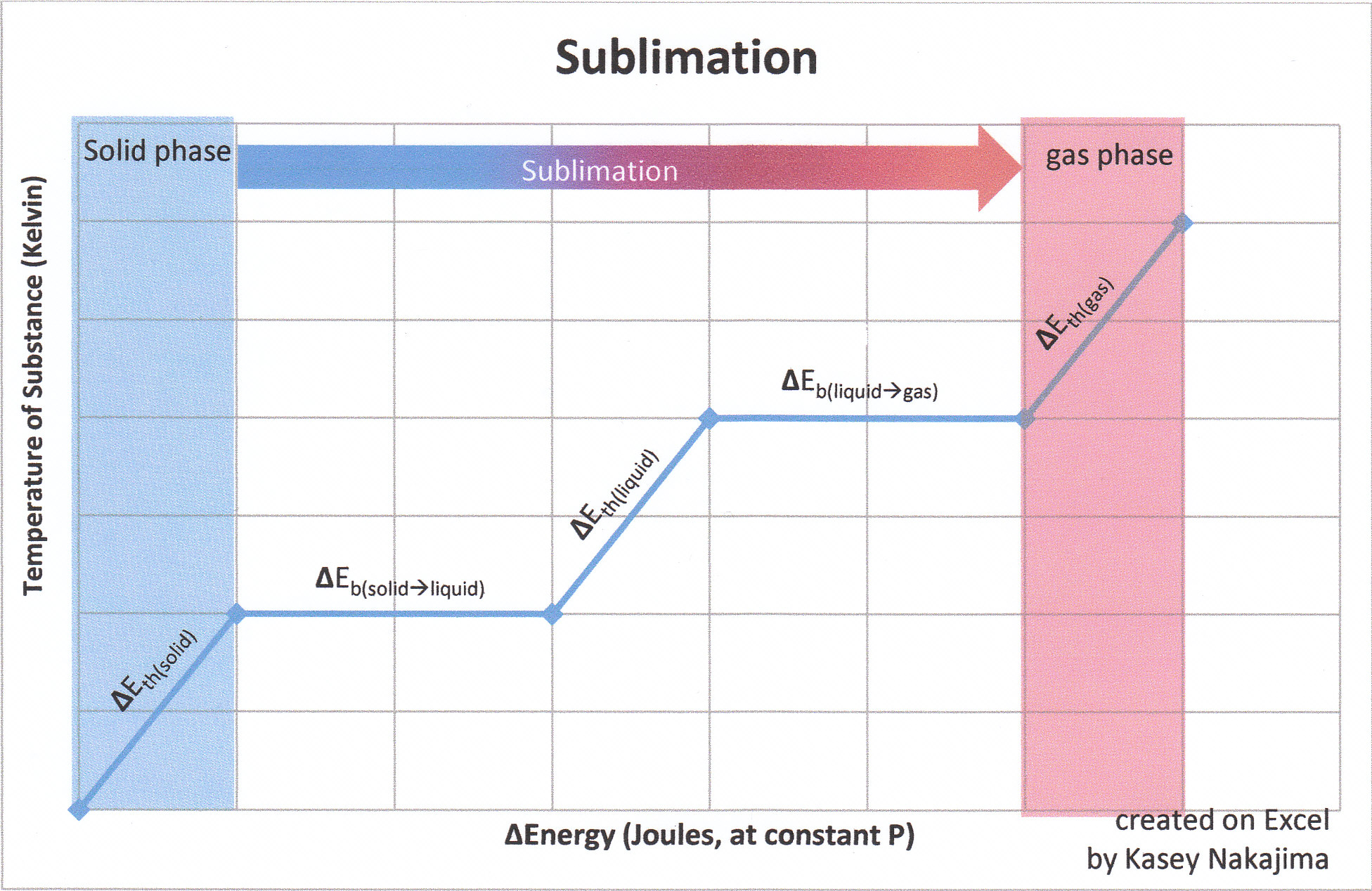
Heat Of Sublimation Chemistry Libretexts
Latent heat also known as latent energy or heat of transformation is energy released or absorbed by a body or a thermodynamic system during a constant-temperature process usually a first-order phase transition.
Is heat absorbed or released in deposition. Absorbed longwave radiation from gases in atmosphere-5. Heat energy is slowly gained by the substance. A At the temperature and pressure at point 4 Yg will spontaneously convert to Ylb At 0 o C and 1200 torr Y exists as a solid.
This would cool 1kg of air by 25oC. C At the pressure and temperature of point 1 Ys will spontaneously convert. Pyometra is considered a serious and life threatening condition that must be treated quickly and aggressively.
These nucleotides are readily absorbed and transported throughout the body to be used by individual cells during nucleic acid metabolism. Absorbed shortwave radiation from the sun-116. Heat is defined in physics as the transfer of thermal energy across a well-defined boundary around a thermodynamic systemThe thermodynamic free energy is the amount of work that a thermodynamic system can perform.
Much more heat is absorbed and conducted through the material. Incoming energy Outgoing energy. Building materials therefore have heat storage capacities.
This proposed rule describes the conditions under which FDA. This heat is then slowly released during the night adding warmth to the urban atmosphere. The Food and Drug Administration FDA or Agency is issuing this proposed rule to put into effect a final monograph for nonprescription over-the-counter OTC sunscreen drug products.
A few examples are neutralization burning a substance reactions of fuels deposition of dry ice respiration solution of sulfuric acid into water and much more. According to the phase diagram given for Compound Y what description is correct. Pyometra is defined as an infection in the uterus.
Units Source Units Source 47. The coating thickness ranges from 50 to 65 µm. Fluorouracil is associated with a low rate of transient serum aminotransferase elevations during therapy and has been implicated in rare cases of clinically apparent acute liver injury.
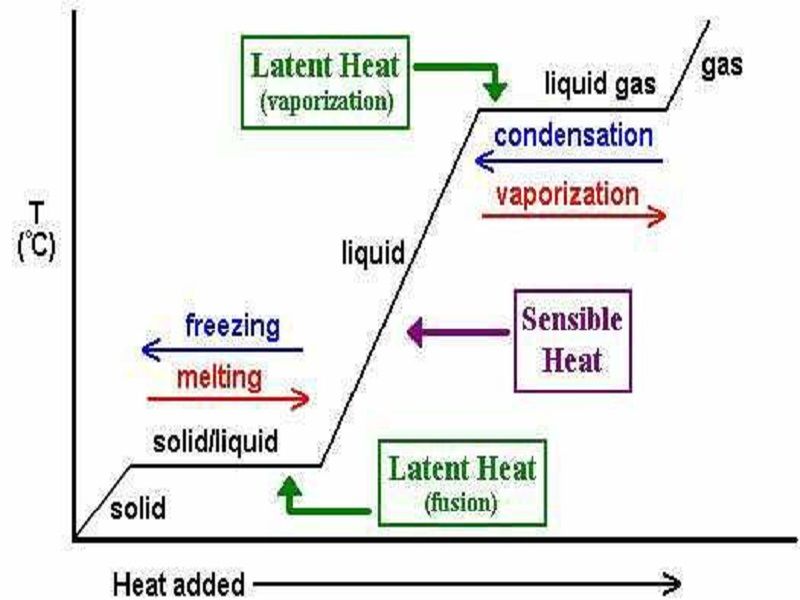
Below are the values in two different scales which are. During evaporation heat energy is absorbedused. Kinetic Energy Potential Energy and a Heating Curve.
Glass and stainless steel to minimise corrosion from acidic condensate deposition. Since Temperature is a measure of Average Kinetic Energy. The deposition efficiency of the n-th generation denoted by η is the percentages of the particles absorbed trapped in this generation of airways relative to the particles released at the inlet surface given by.
Difference Between Endothermic and Exothermic Reactions. At the earths surface - Energy absorbed is balanced with the energy released. A cross-section image of the as-sprayed alumina coating is displayed in Figure 3.
Heat energy is maintained by the substance. In a secondary process the rest of the heat is released to the surroundings at a lower temperature because the heat engine is not 100 efficient. Basics of radiation addresses energy from a source that travels through material or space.
To complete the heat budget the heat that is absorbed by the atmosphere either directly from solar radiation or as a result of conduction radiation and latent heat is eventually radiated back into space Figure 811. However it is most common in older cats. Once a drug has traversed the stratum corneum the next layer is easier to cross and subsequently the drug can reach the capillary vessels to be absorbed Mitragotri et al.
Heat energy is quickly absorbed by the substance. Click here for Calculations and Heating Curves. There is no temperature change during a phase change thus there is no change in the kinetic energy of the particles in the material.
As shown in Figure 2 a refrigerator can be thought of as a heat engine in reverse. Light and heat are types of radiation. The energy released comes from the potential energy stored in the bonds between the particles.
The kind of radiation discussed here is called ionizing radiation because it has enough energy to remove an electron from an atom making that atom an ion. Heat is absorbed by ice when it melts and heat is released when ice forms and these phase changes transfer heat between the oceans and the atmosphere. This keeps urban climates relatively high and the contrast between the urban environment and the rural fringe is greatest at night.
This results in heat being absorbed at the hot section of the pipe. Erbium provided pure ablation while CO 2 allowed heat deposition for skin tightening. Removal of heat by convection rising warm air.
Enthalpy is a thermodynamic potential designated by the letter H that is the sum of the internal energy of the system U plus the product of pressure P and volume V. Heat absorbed or released as the result of a phase change is called latent heat. Longwave radiation emitted by the surface.
Absorbed photons can produce thermal mechanical. Why does it occur. Pyometra may occur in any sexually intact young to middle-aged cat.
Typically the cat has been in heat within the previous 4 weeks. When transparent the built up photons get released just for a few nanoseconds and blocked again to get rebuilt up. Before the deposition of the coatings all powders were dried to prevent moisture from being absorbed during storage.
It takes 600 calories of heat to convert 1 gramme of water to a vapour. It releases energy by light or heat to its surrounding. Latent heat can be understood as energy in hidden form which is supplied or extracted to change the state of a substance without changing its temperature.
In a heat engine a working substance absorbs heat at a high temperature and converts part of this heat to work. As heat is absorbed by electrons at the top junction and that heat energy is carried away from the junction via the Peltier effect the cold-side chip temperature decreases. This skin permeabilization method may be useful for avoiding the multiple use of needles for example for delivery of heparin or insulin through the skin Smith 2008.
At the center of the problem-solving strategy is the recognition that the quantity of heat lost by the water Q water equals the quantity of heat gained by the metal Q metalSince the m C and ΔT values of the water are known the Q water can be calculated. Compared to the previous problem this is a much more difficult problem. The majority of heat is released into the atmosphere.
Fluorouracil 5-FU is a pyrimidine analogue used as an antineoplastic agent to treat multiple solid tumors including colon rectal breast gastric pancreatic ovarian bladder and liver cancer. The exothermic reaction is the opposite of an endothermic reaction. The electrons carrying heat energy travel to the bottom connector where excess heat is released through an external heat.
In fact this problem is like two problems in one. The heat used passes into the water and is known as latent heat latent heat transfer. Which best describes the energy change that takes place during deposition.
The system transfers heat from the exhaust gases to the cool water that is flowing through the pipes of the heat exchanger. Deposition was used to grow. The remaining 60 percent of the energy released from catabolic reactions is given off as heat.
A 1 2 and 3 b 4 5 and 6 c 1 and 2 only d 4 and 6 only e some other combination 11.

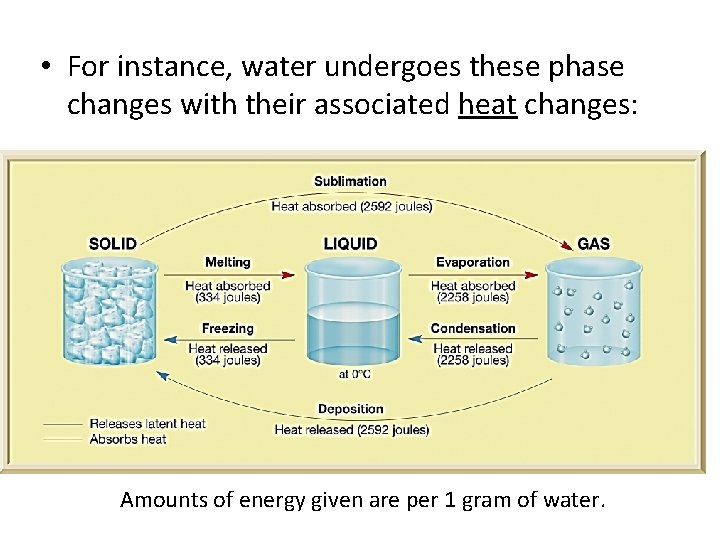
File Heat Absorbed And Realeased Of Water Png Wikimedia Commons
Heat And Temperature Almost Every Chemical Reaction Is
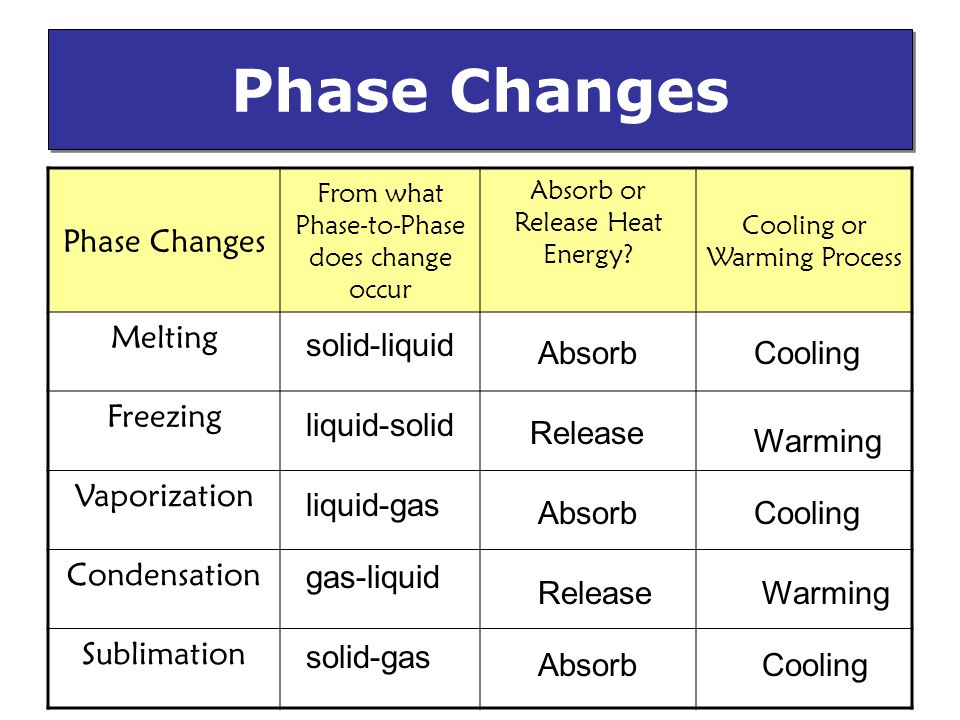
Phase Changes Physical Change Of Matter From One Phase To Another Due To A Transfer Of Energy Ppt Video Online Download

Geog 1112 Weather And Climate Atmospheric Moisture And Precipitation Ppt Download
Thermal Properties Of Matter

Latent Heat Loss An Overview Sciencedirect Topics
Enthalpy Of Fusion Wikipedia
Climate Science Investigations South Florida Energy The Driver Of Climate
